Surfactants, short for surface-active agents, are compounds that lower the surface tension between two substances, such as between a liquid and a solid, or between two liquids. They are unique molecules with two distinct parts: a hydrophobic tail (water-repelling) and a hydrophilic head (water-attracting). This dual nature allows surfactants to interact with both oil and water, making them essential in a wide range of products, from soaps to industrial cleaners.
Surfactants work by disrupting the interactions between molecules on the surface of liquids. In cleaning applications, for instance, the hydrophobic tail binds with oils and grease, while the hydrophilic head binds with water. This enables the surfactant to emulsify and remove dirt and grease from surfaces effectively.
Surfactants come in several types, each suited to different applications.
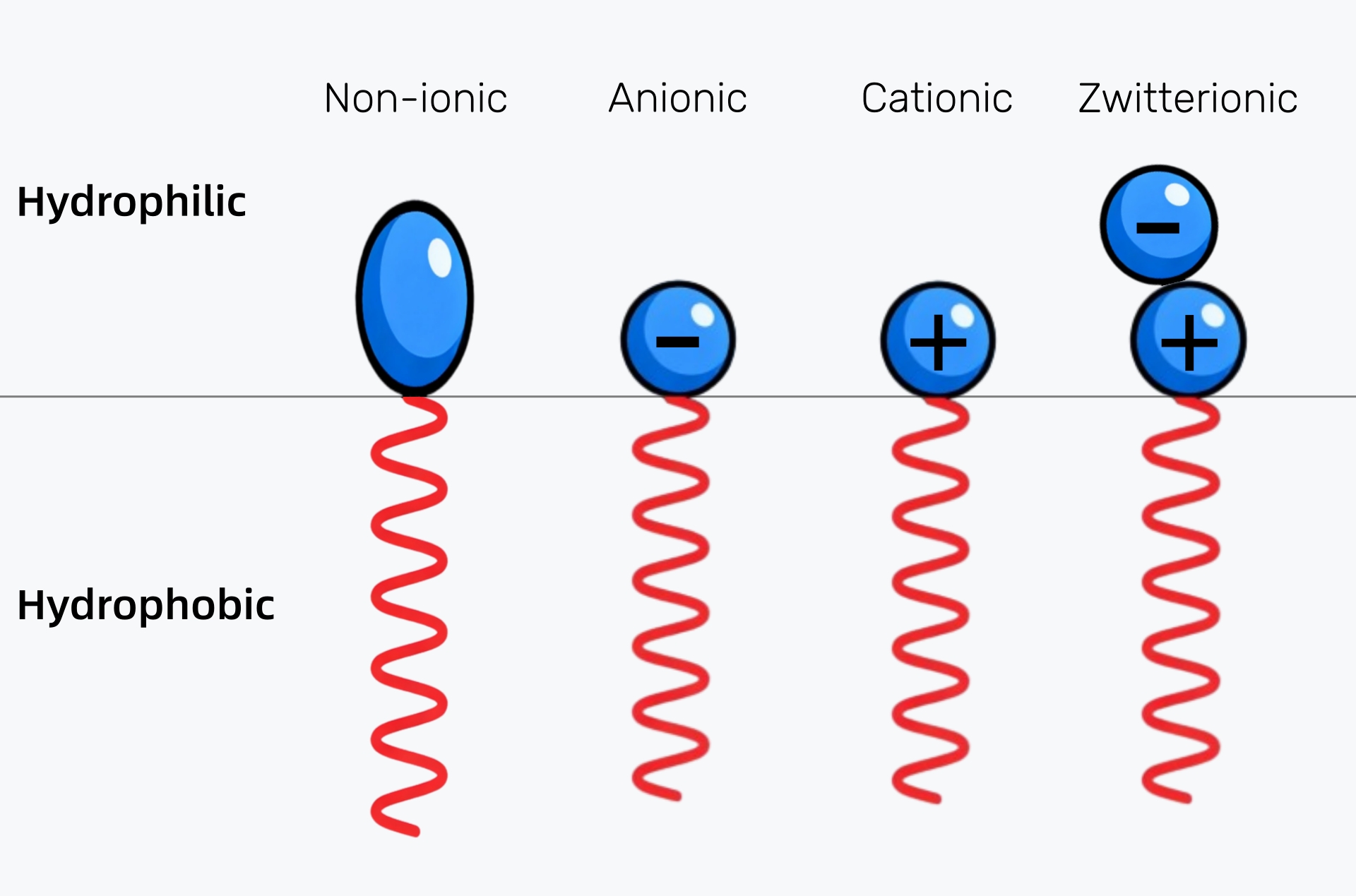
The most common are:
Each type of surfactant serves a specific purpose, ensuring that products perform optimally in different environments.

Surfactants are the cornerstone of most cleaning products because they significantly enhance the removal of dirt, grease, and contaminants. The main benefit of surfactants in cleaning is their ability to emulsify oils and dirt, allowing them to be suspended in water and washed away.
In industrial settings, surfactants are used in formulations for cleaning machinery, removing oils from surfaces, or even in washing textiles in large-scale laundromats. Their ability to lower the surface tension helps cleaning solutions penetrate hard-to-reach surfaces, making them effective in both residential and commercial cleaning.
Moreover, surfactants contribute to reducing the need for high temperatures or harsh chemicals, offering an eco-friendly alternative to conventional cleaning methods.
While surfactants are crucial for cleaning and personal care, their safety and environmental impact are important considerations. Many surfactants, especially synthetic ones, can pose risks to aquatic life if not properly managed, due to their ability to disrupt biological membranes in living organisms.
To mitigate these risks, many industries have shifted to using biodegradable surfactants that break down more easily in the environment. Additionally, non-toxic surfactants are being developed for use in personal care products to minimize irritation to skin and eyes.
Despite their utility, it’s essential for manufacturers to use surfactants responsibly, adhering to regulations and ensuring that their products are safe for consumers and the environment.
The debate between natural and synthetic surfactants has gained significant attention, particularly in consumer goods. Natural surfactants, derived from plant-based sources like coconut oil or corn, are often preferred for products that claim to be eco-friendly and gentle on the skin. They tend to be biodegradable and have a lower environmental impact.
On the other hand, synthetic surfactants are typically more cost-effective and more stable in a variety of formulations. They can offer higher cleaning performance, which is why they dominate in industrial and commercial cleaning products. However, some synthetic surfactants may have harsher effects on sensitive skin or the environment, prompting a shift toward more sustainable options.
Ultimately, the choice between natural and synthetic surfactants depends on the product's purpose, the target market, and the brand’s sustainability goals.